Gene therapy for hemophilia represents a groundbreaking advancement in treating this genetic disorder, particularly hemophilia B, where patients suffer from a deficiency of clotting factor IX. In February 2025, Terence Blue became the first individual in New England to receive Hemgenix, a pioneering gene therapy approved by the FDA in 2022, specifically designed to address the root cause of hemophilia. This innovative treatment harnesses the power of gene and cell therapies to effectively introduce a corrected gene into the patient’s liver, where clotting factors are produced. With therapies like Hemgenix, individuals can potentially alleviate years of dependence on routine clotting factor injections, marking a significant shift towards enhanced quality of life. As research continues to advance, the promise of new gene therapy options for hemophilia could redefine patients’ experiences, offering hope where traditional treatments have fallen short.
Innovations in treating hemophilia, particularly hemophilia B, have begun to reshape the landscape of patient care. The advent of therapies such as Hemgenix reflects a growing interest in genetic interventions aimed at resolving the underlying genetic issues that cause these bleeding disorders. This new gene therapy approach involves introducing a functioning gene that promotes the production of essential clotting factors, significantly simplifying treatment regimens. As the field of gene and cell therapies continues to evolve, individuals facing hemophilia may find themselves on the cusp of a transformative era, moving away from conventional treatment approaches. The ongoing developments in this area symbolize not just scientific progress but also a compelling shift in the lives of those living with hemophilia.
Advancements in Gene Therapy for Hemophilia
Gene therapy has emerged as a revolutionary treatment strategy for hemophilia, particularly hemophilia B. One of the most significant breakthroughs in this field is the introduction of Hemgenix, a therapy that promises to address the underlying genetic cause of the disorder rather than merely managing its symptoms. Unlike traditional methods that require regular infusions of clotting factors, Hemgenix aims to provide a long-term solution by delivering a corrected gene that instructs the body to produce the missing clotting factor IX. This innovative approach not only enhances the quality of life for patients but also reduces the dependency on frequent medical interventions.
Moreover, the lasting effects of Hemgenix could potentially diminish or even eliminate the need for ongoing treatment, offering a new lease on life to individuals like Terence Blue, who have managed their hemophilia for decades. As researchers continue to explore similar gene and cell therapies, the hope is to replicate this success across various forms of hemophilia and other blood disorders. With an increasing number of gene therapies being approved, there is optimism that patients will experience fewer spontaneous bleeds and improve their overall well-being.
Challenges and Hurdles in the Adoption of Gene Therapies
While the advancements in gene therapy for hemophilia are promising, the real-world application of these therapies faces significant challenges. One major hurdle is the high cost associated with treatments like Hemgenix. The price tag of $3.5 million for a single administration sparks concerns regarding access and affordability for patients, many of whom already face financial burdens due to regular treatment needs. The market dynamics, combined with limited patient numbers, make it challenging for pharmaceutical companies to justify the substantial investment in developing these therapies.
Additionally, the acceptance of gene therapy among patients and healthcare providers is critical for its success. Some patients may have reservations about introducing foreign genes into their bodies, leading to hesitance in opting for such treatments. Education and awareness campaigns are essential to help patients understand the benefits and risks involved, as well as the transformative potential of gene therapies. As seen in other cases, such as the withdrawal of Pfizer’s Beqvez gene therapy, market pressures can lead to uncertainty about the longevity of these therapies in the healthcare landscape.
The Impact of Hemgenix on Quality of Life
Hemgenix represents a monumental shift in how hemophilia B is treated and managed. For patients like Terence Blue, the therapy not only alleviates the physical burden of living with a chronic condition but also significantly enhances their quality of life. With the potential to produce sufficient levels of factor IX, patients can enjoy everyday activities without the constant worry of spontaneous bleeding or the complications that come from managing a bleeding disorder. Reports suggest that individuals treated with Hemgenix often experience fewer hospital visits and greater freedom to partake in physical activities.
Furthermore, an improved quality of life extends beyond physical health. Patients report a profound psychological impact, as the fear of injury diminishes with the newfound ability to heal faster. The psychological relief from constant injections and the anxiety tied to managing hemophilia represents a new chapter for many individuals. As the therapy gains acceptance and more patients experience these benefits, there’s hope for a future where hemophilia B is less of a life-altering burden and more of a manageable condition.
Market Dynamics Affecting Gene and Cell Therapies
The advent of gene therapies like Hemgenix has reignited hope for patients with hemophilia, but market dynamics are proving challenging for the widespread adoption of these advanced treatments. Drugmakers must navigate a complex terrain that includes high development costs, regulatory approval hurdles, and the need for market acceptance. With the market still adjusting to the realities of pricing and patient access, it’s crucial for stakeholders—from pharmaceutical companies to healthcare providers—to align their interests to ensure these therapies reach the patients who need them most.
Market acceptance does not just involve economic feasibility but extends to the perception of treatment safety and efficacy among patients and clinicians alike. For gene therapy to thrive, it is essential for ongoing education and transparency regarding treatment benefits, potential side effects, and long-term outcomes. The healthcare ecosystem must evolve alongside technological advancements to help patients make informed decisions about their treatment options—in turn driving greater acceptance and utilization of innovative therapies.
The Future of Hemophilia Management with Gene Therapy
As the field of gene therapy for hemophilia evolves, the future looks promising. Hemgenix’s recent approval has catalyzed renewed interest in gene and cell therapies for not just hemophilia, but a range of blood disorders. The success stories emerging from early adopters showcase the potential for significant advancements in patient care, aiming to transition from reactive management strategies to proactive, long-term treatments. This shift could not only improve individual outcomes but also result in broader healthcare cost savings by reducing the need for ongoing treatments and hospitalizations.
Moreover, ongoing research is crucial in driving innovation in this space. As scientists better understand the genetic underpinnings of various hemophilia types, including hemophilia A and B, new therapies will be developed. With CRISPR and other gene-editing technologies at the forefront, the possibility of personalized medicine approaches tailored to individual patients will become more feasible. Ultimately, advancing gene therapy could lead to a day where hemophilia is no longer a limitation to an active, fulfilling life.
Patient Experiences with Hemogenix: A Real-Life Perspective
Understanding the patient experience with Hemgenix provides invaluable insights into the real-world impact of this novel gene therapy. For patients like Terence Blue, receiving the treatment marked a significant turning point in their lives. After years of managing hemophilia with regular factor infusions and careful lifestyle adjustments, experiencing a new sense of freedom and normalcy is life-changing. The emotional aspects associated with living with a chronic condition can often be overlooked, but they play a crucial role in treatment decision-making and overall satisfaction.
As Terence shared, the emotional toll of dealing with daily needles and the constant vigilance surrounding his condition had been daunting. Transitioning to a gene therapy that offers a chance at not only improving physical health but also alleviating that emotional burden can be profoundly transformative. The collective experiences of patients will be vital in guiding future therapies, shedding light on essential factors such as treatment adherence, lifestyle modifications, and overall satisfaction.
Understanding Hemophilia B and Its Treatment Options
Hemophilia B, a genetic disorder characterized by the deficiency of clotting factor IX, is one of the two main types of hemophilia. This condition affects roughly 15 percent of individuals with hemophilia and poses unique challenges and risks, including a propensity for internal bleeding and joint damage. For those living with hemophilia B, traditional treatment options have revolved around regular infusions of clotting factor to prevent bleeds, which can be both burdensome and impractical for long-term management.
However, with advances in gene therapy, such as the introduction of Hemgenix, the landscape of treatment options is changing. The ability to potentially cure or significantly reduce the impact of hemophilia B through a single infusion provides hope and encourages research into more comprehensive treatment strategies. Continued exploration of gene and cell therapies is crucial to evolve the standard of care, making it imperative for researchers and healthcare providers to remain informed about the latest innovations in treating hemophilia.
Empowering Patients Through Education and Resources
Educating patients about their condition and emerging treatment options is vital for ensuring informed decision-making. As gene therapies become more prevalent, it’s essential for individuals diagnosed with hemophilia to understand how these treatments work, their potential benefits, and any associated risks. Organizations dedicated to hemophilia care should work collaboratively with healthcare providers to develop accessible resources that educate patients about advancements like Hemgenix, aiming to empower them to take an active role in their health care.
Equipping patients with knowledge not only enhances their understanding but also fosters open dialogues with healthcare providers. When patients feel informed and supported, they are more likely to engage in discussions about their treatment options and participate in clinical decision-making. This level of engagement can ultimately lead to better adherence to treatment plans and improved health outcomes for those living with hemophilia.
The Role of Advocacy in Advancing Hemophilia Treatment
Advocacy plays a critical role in the development and accessibility of treatments for hemophilia. Organizations and advocacy groups focus on raising awareness about the challenges faced by individuals living with hemophilia, as well as promoting research and funding for new therapies like Hemgenix. Advocacy efforts are crucial in ensuring that the needs and voices of patients are heard, which can drive policy changes needed to support access to cutting-edge treatments.
Furthermore, effective advocacy can lead to greater public awareness and understanding of hemophilia, pushing for necessary resources and support systems that enhance patient care. By bridging the gap between patients, healthcare providers, and policymakers, advocacy organizations can facilitate a more supportive environment for innovative therapies to thrive, ultimately improving the lives of those affected by hemophilia.
Frequently Asked Questions
What is gene therapy for hemophilia, and how does it work?
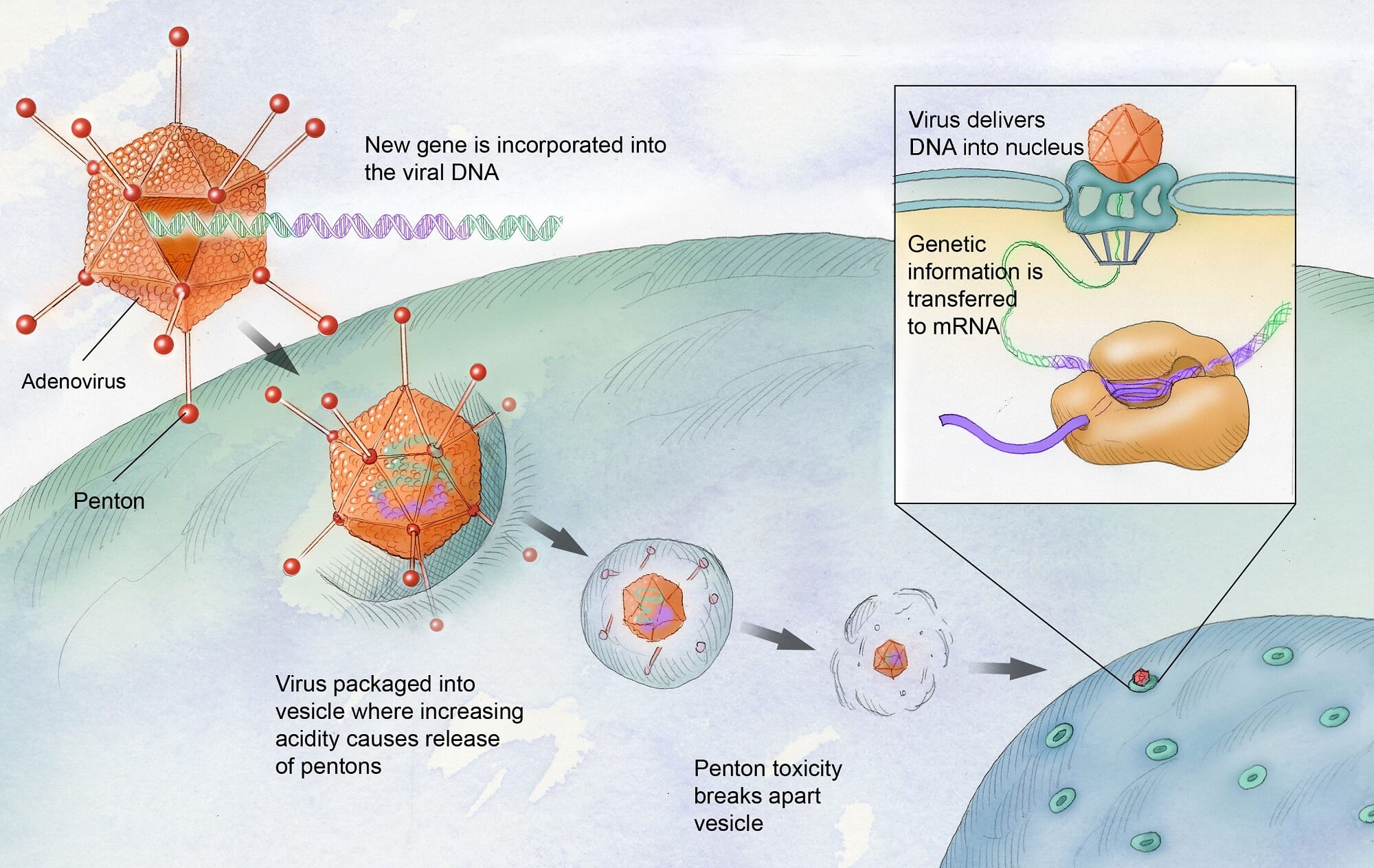
Gene therapy for hemophilia aims to treat the genetic cause of the disorder by introducing corrected genes into the patient’s body. In hemophilia B, for example, a virus is engineered to deliver a healthy copy of the gene responsible for producing clotting factor IX directly into the liver. This can help restore normal clotting function, significantly reducing or eliminating the need for frequent factor injections.
How does Hemgenix fit into the new gene therapy landscape for hemophilia?
Hemgenix is a groundbreaking gene therapy for hemophilia B that was approved by the FDA in November 2022. It represents a significant advance in gene and cell therapies, promising long-lasting effects by enabling the liver to produce sufficient levels of clotting factor IX. By addressing the underlying genetic defect, Hemgenix provides a potential cure alternative to traditional clotting factor treatments.
What are the benefits of gene and cell therapies for hemophilia patients?
Gene and cell therapies, like those for hemophilia, can offer substantial benefits, including the potential for sustained production of clotting factors without the need for regular injections. These therapies aim to improve the quality of life for patients by reducing the risk of bleeding episodes and the burden of ongoing treatments, thereby promising to transform hemophilia management.
What should patients expect during the administration of gene therapy like Hemgenix?
Patients receiving Hemgenix can expect a relatively straightforward outpatient procedure. The therapy is delivered via an infusion that takes approximately two hours under close monitoring by healthcare professionals. After administration, patients may experience mild side effects but typically require no extensive hospitalization.
Is gene therapy for hemophilia considered a cure?
While gene therapy for hemophilia, such as Hemgenix, is not yet universally labeled as a cure, it offers hope for long-term remission. Initial clinical trials show that many patients treated with Hemgenix maintain therapeutic levels of clotting factors for extended periods, significantly improving their quality of life and reducing therapy reliance.
What are the financial considerations for gene therapy in hemophilia treatment?
Gene therapies for hemophilia, like Hemgenix, come with high price tags, often exceeding millions of dollars. However, insurance negotiations and varying patient accessibility can influence costs. These financial considerations are crucial as they impact the wide adoption of these potentially life-changing therapies, as evidenced by the market challenges faced by past gene therapies.
What advancements in safety and efficacy do gene therapies for hemophilia offer?
Recent advancements in gene therapies for hemophilia have improved safety profiles and efficacy. Techniques like using safer viral vectors for gene delivery and optimizing the genetic modifications have increased the reliability and effectiveness of treatments, making them more promising for long-term management of hemophilia.
How does hemophilia B differ from hemophilia A in terms of gene therapy?
While both hemophilia A and B are inherited bleeding disorders, they differ in the specific clotting factor that is lacking: hemophilia A involves factor VIII, and hemophilia B involves factor IX. Gene therapy approaches like Hemgenix specifically target the genetic mutation related to hemophilia B, thus requiring different strategies tailored to each type for effective treatment.
What are the long-term effects of receiving a gene therapy for hemophilia?
The long-term effects of receiving gene therapy for hemophilia, particularly with treatments like Hemgenix, may include sustained production of clotting factors which can lead to a significant reduction in bleeding episodes and dependency on external factor injections. Clinical trials have demonstrated that many patients remain stable for several years post-treatment, although ongoing monitoring is essential.
What role do gene and cell therapies play in the future treatment of hemophilia?
Gene and cell therapies are poised to revolutionize the future treatment of hemophilia by offering durable, potentially curative options. As research continues and more therapies are developed, patients may expect improved outcomes, less frequent treatments, and an overall enhanced quality of life.
| Key Points | Details |
|---|---|
| Terence Blue’s Experience | First patient in New England to receive Hemgenix gene therapy for hemophilia B. |
| Gene Therapy Overview | Hemgenix aims to correct the mutation causing hemophilia and boost clotting factor IX production. |
| Historical Context | Gene therapies like Hemgenix represent a significant advancement in hemophilia treatment, providing hope for greater independence from regular factor infusions. |
| Upcoming Challenges | High costs and market acceptance are barriers; Blue’s treatment costs about $3.5 million. |
| Future Potential | Initial results show promise, with many patients potentially not needing regular factor IX treatments post-therapy. |
Summary
Gene therapy for hemophilia is transforming treatment options for individuals like Terence Blue, who underwent therapy that offers the possibility of living with less reliance on daily factor injections. This innovative approach seeks to correct the genetic mutation behind hemophilia, significantly improving patients’ lives and advancing the field of medicine. As more breakthroughs emerge, gene therapy promises to bring us closer to a potential cure, providing hope and improved quality of life for those affected by this condition.