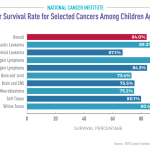
The recent medical research funding cuts have sent shockwaves through the healthcare landscape, jeopardizing critical advancements in patient safety. With over $2 billion in federal grants from the National Institutes of Health (NIH) halted, institutions like Harvard are facing unprecedented disruptions in their research efforts. This freeze not only halts clinical trials but also impedes the essential IRB oversight necessary to protect participants’ rights and well-being. As resources dwindle, the consequences for patient safety could be dire, with the potential for increased risks in ongoing studies. The implications of these funding cuts extend beyond academia; they affect every citizen who relies on the safety and efficacy of medical advancements derived from research.
The recent reductions in funding for medical research, often referred to as budget cuts, represent a significant concern in the realm of healthcare innovation. Investigations into therapies and treatments, crucial for patient care and public health, are at risk of stagnation due to financial constraints imposed by federal funding restrictions. These funding cuts also hinder the vital regulatory frameworks, such as Institutional Review Board (IRB) oversight, that are fundamental in ensuring the ethical treatment of research participants. Academic institutions renowned for pioneering studies, like Harvard, are grappling with diminished resources, posing challenges to the integrity and safety of clinical trials. Without adequate financial support, the potential for groundbreaking discoveries that enhance patient safety becomes increasingly bleak.
Understanding the Impact of Medical Research Funding Cuts
The Trump administration’s decision to freeze over $2 billion in medical research funding, specifically affecting institutions like Harvard, has sent shockwaves through the healthcare research community. Such funding cuts can have far-reaching consequences, particularly for clinical trials that play a vital role in developing new treatments and improving existing ones. As research initiatives come to a standstill, the implications for patient safety and progress in medical advancements become evident. Researchers and institutions that rely on these resources are left scrambling to maintain their projects, while the health and safety of patients participating in these trials hang in the balance.
Medical research funding is critical, not only for advancing scientific knowledge but also for ensuring that established safety protocols are maintained. With decreased funding, the ability to conduct thorough Institutional Review Board (IRB) assessments diminishes, which amplifies the risks associated with medical trials. This situation undermines trust in the healthcare system, particularly among vulnerable populations who are often the subjects of clinical trials. As funding cuts persist, the long-term consequences could deter potential participants from volunteering in research, ultimately stalling progress essential for medical breakthroughs.
The Role of IRB Oversight in Protecting Patients
Institutional Review Boards (IRBs) serve as the backbone of ethical research practices. Their primary responsibility is to ensure that all aspects of human research comply with ethical standards and regulatory requirements, thereby safeguarding patient welfare. By conducting thorough reviews of study protocols—ranging from informed consent processes to risk mitigation strategies—IRBs uphold the integrity of medical research. This oversight is especially vital in the context of complex clinical trials where participant safety can be at significant risk if not adequately managed.
Funding cuts jeopardize IRB functions and their ability to oversee multiple-site studies effectively. With a significant reduction in resources, IRBs may struggle to adequately monitor research initiatives and ensure compliance with regulations. This shortfall can lead to increased instances of protocol violations, exacerbating potential harm to patients. Given the historical context of ethical breaches in medical research, it becomes imperative that IRBs receive the necessary funding to continue their vital role in protecting the rights and safety of research participants.
Consequences for Clinical Trials and Patient Safety
The repercussions of funding cuts extend deeply into the fabric of clinical trials, which are essential for validating new treatments and ensuring patient safety. A lack of funding inhibits the execution of rigorous trial designs and diminishes the resources available for patient monitoring and care. As a result, research institutions may find themselves challenged to uphold the high standards required for clinical trials, potentially placing participants at risk due to inadequate oversight and support.
Moreover, the halting of ongoing trials can lead to a ripple effect that affects not only current participants but also future research viability. If trials are interrupted, the accumulated data and findings may become obsolete, forcing researchers to restart processes, wasting both time and precious resources. Such setbacks can create a chilling effect on research participants’ willingness to engage in future studies, as trust in the system erodes amid safety concerns stemming from inadequate funding and oversight.
The Role of NIH Funding in Research Integrity
The National Institutes of Health (NIH) plays a crucial role in supporting health research, providing essential funding that helps maintain rigorous standards in IRB oversight. NIH funding is not just financial assistance; it represents a commitment to ethical research practices and the protection of human subjects involved in studies. The guidelines established for NIH-funded research underscore the importance of ethical practices and patient safety, illustrating how financial support directly correlates with enhanced research integrity.
However, if federal funding sources like the NIH are cut, the integrity of research may suffer significantly. Reduced funding jeopardizes the ability to conduct comprehensive IRB reviews and oversights, leading to potential compromises in patient safety. The consequences of diminished NIH funding ripple through the research community, affecting everything from study design to participant recruitment, ultimately impacting vital medical advancements and the trust between researchers and the communities they serve.
Community Impact of Research Funding Cuts
When funding for medical research is curtailed, the effects resonate far beyond the confines of research institutions. Communities that rely on innovative treatments and trusted clinical trials may find themselves at a disadvantage, resulting in a decreased quality of healthcare access. Marginalized and underserved populations often rely on clinical trials to receive cutting-edge therapies, making their exclusion from these services particularly detrimental. As funding dwindles, the gap between available treatments and community needs widens, exacerbating health disparities.
Additionally, the connection between researchers and community members is critical for fostering trust and collaboration. When funding cuts stifle research efforts, the resulting delays and disruptions can lead to skepticism about clinical trials and the healthcare system as a whole. This mistrust can discourage individuals from participating in future studies, thus jeopardizing the progress needed to enhance public health and safety. Engaging communities is essential: it not only bolsters the recruitment process for clinical trials but also reinforces the social contract of trust between researchers and participants.
Historical Lessons on Ethical Oversight in Research
The establishment of IRBs was driven by the need to address the horrors of past medical research abuses, including unethical experiments that violated individuals’ rights. Historical cases like the Tuskegee Syphilis Study highlighted the dire need for oversight to protect human subjects. As the ethical landscape of research evolved, regulatory bodies emerged to ensure that the lessons from these past mistakes were not forgotten. This scrutiny is particularly relevant today as the integrity of clinical trials hangs in balance due to funding cuts, and the dangers of repeating history loom larger.
By analyzing historical events, it becomes clear that ethical oversight must remain a top priority in the face of funding challenges. The unfortunate reality is that reduced financial support can compromise the thoroughness of IRB reviews, increasing the likelihood of ethical violations. By understanding the lessons of the past, the research community can advocate for continued funding to safeguard the rights of participants and uphold the ethical standards required in medical research.
The Importance of Collaborative Research Models
Collaborative research models have become essential in conducting efficient and effective medical research, especially as the healthcare landscape becomes increasingly complex. Initiatives like the SMART IRB were designed to streamline the processes associated with multisite studies, allowing for a single IRB to oversee multiple institutions. This innovative approach reduces redundancies, accelerates timelines, and fosters a collaborative spirit among academic and healthcare institutions. However, with significant funding cuts, these collaborative frameworks are in jeopardy, which could stifle innovation and delay critical research meant to benefit patient health.
Without sufficient resources, the ability to collaborate effectively diminishes, leading to disjointed research efforts and inefficiencies. As hospitals and universities grapple with reduced funding, the landscape may regress to siloed approaches that hamper progress. The consequences for patient safety are severe; fragmented research not only delays critical findings but also limits the ability of research teams to ensure comprehensive oversight and protocol adherence, essential for protecting participants involved in clinical trials.
Navigating the Future of Medical Research Funding
The outlook for medical research funding is precarious, particularly in light of administrative decisions that impact the allocation of federal resources. As institutions face funding cuts, it becomes crucial to advocate for policies that prioritize patient safety and research integrity. Stakeholders, including researchers, healthcare professionals, and community members, must come together to highlight the essential role of funding in maintaining robust oversight systems like IRBs, which are necessary for protecting research participants.
Moving forward, it will be vital to restore and enhance funding for medical research, not just to revitalize current studies but to enable future innovations. Public pressure and advocacy can play significant roles in lobbying for increased NIH funding and support for collaborative initiatives. The safety and well-being of research participants depend on the ability of the scientific community to unite and ensure that medical research can continue to thrive, paving the way for breakthroughs that enhance public health.
Frequently Asked Questions
What are the potential impacts of medical research funding cuts on patient safety?
Medical research funding cuts can significantly jeopardize patient safety by disrupting essential oversight functions carried out by Institutional Review Boards (IRBs). Without adequate funding, IRBs may struggle to ensure compliance with safety regulations, potentially allowing harmful practices to occur. Cuts to funding can lead to delays or interruptions in clinical trials, compromising the rigorous monitoring needed to protect participants’ welfare.
How does NIH funding support essential IRB oversight in medical research?
NIH funding is crucial for supporting IRB oversight which is imperative in protecting the rights and safety of participants in medical research. These funds enable IRBs to review and oversee research proposals, ensuring compliance with ethical standards and safety protocols. Reduction in NIH funding can weaken this oversight, risking the integrity of clinical trials and the safety of research participants.
What role does Harvard research play in ensuring patient safety amid funding cuts?
Harvard research contributes significantly to patient safety by facilitating a robust framework for IRB oversight through initiatives like SMART IRB. Funding cuts can hinder Harvard’s ability to advance collaborative research and maintain necessary oversight functions, adversely affecting the safety and welfare of individuals participating in clinical trials conducted across multiple sites.
How will medical research funding cuts affect clinical trials and patient safety?
Cuts to medical research funding can severely affect clinical trials by halting new studies, delaying ongoing research, and limiting collaboration among institutions. This disruption poses risks to patient safety, as it can prevent timely assessments of new therapies and innovative treatments designed to protect participants and enhance public health.
What historical events highlight the importance of IRB oversight in light of medical research funding cuts?
Historical events such as the Tuskegee Syphilis Study underscore the critical importance of IRB oversight in medical research. Funding cuts threaten the ongoing evolution and enforcement of ethical research practices established in response to past abuses. Maintaining robust IRB oversight is essential to ensure that modern clinical trials prioritize patient safety and uphold ethical standards.
In what ways can public skepticism towards medical research increase due to funding cuts?
Medical research funding cuts can exacerbate public skepticism by interrupting studies and compromising the transparency of research practices. When IRBs struggle to perform their oversight responsibilities due to lack of funding, it leads to mistrust among communities and potential participants, jeopardizing future recruitment and collaboration essential for advancing patient safety in clinical trials.
| Key Points | Details |
|---|---|
| $2 Billion Funding Cut | The Trump administration froze over $2 billion in federal research grants, impacting patient safety in medical studies. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure compliance with regulations to protect the rights and welfare of research participants. |
| Impact of Cuts on IRBs | Funding cuts threaten the functionality of IRBs, risking ethical oversight and protections for study participants. |
| Research Delays | Numerous studies are experiencing significant delays and many sites have been barred from joining collaborative research due to funding cuts. |
| Public Trust | Cuts may reinforce public skepticism regarding the safety and efficacy of medical research efforts. |
Summary
Medical research funding cuts severely hinder efforts to ensure patient safety in clinical studies. The substantial freeze on federal grants has disrupted critical oversight mechanisms that protect participants’ rights and welfare. As a consequence, many research initiatives face delays, prompting concerns about the ethical conduct of research and public trust in the medical research community. With ongoing cuts, it is essential to reassess funding policies to safeguard not only the integrity of the research process but also the health and safety of individuals involved in these vital clinical trials.