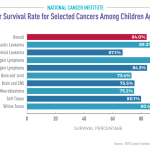
The impact of funding cuts on medical research has been profound, jeopardizing critical advances in healthcare and patient safety in research. Recent NIH funding cuts have frozen over $2 billion in federal research grants, disrupting essential studies that protect the rights and well-being of participants. These funding limitations hinder the ability of Institutional Review Boards (IRBs) to fulfill their roles effectively, as they depend on adequate resources to oversee clinical research ethics and ensure compliance with regulations. As serious health challenges like Alzheimer’s disease demand urgent attention, curtailed research funding can stifle innovation and exacerbate public distrust in scientific inquiry. Without the necessary financial backing, the entire ecosystem of medical research that benefits countless individuals is at risk of collapse.
The repercussions of budget reductions in the realm of health studies are significant, directly affecting the landscape of experimental medicine and clinical trials. As financial support dwindles, research initiatives aimed at advancing treatments or safeguarding patient rights face unprecedented challenges. The current crisis over research allocations raises questions about the integrity of institutions responsible for overseeing the safety of human participants. Additionally, these funding shortages impede collaborative efforts among universities and hospitals, directly impacting the ethical oversight processes crucial for maintaining trust in medical experimentation. As legislative measures and policy decisions shift, the future of medical research hangs in the balance, urging us to re-examine how we prioritize health-related investments.
The Consequences of Funding Cuts on Medical Research
Funding cuts can severely disrupt the landscape of medical research, particularly in areas focused on patient safety and ethical oversight. As seen with the recent stop-work order affecting Harvard’s SMART IRB program, the loss of over $2 billion in federal grants has stalled numerous critical studies. This interruption not only delays the research itself but also halts the institutional review boards’ (IRBs) ability to perform their essential duties. IRBs are charged with ensuring participant rights and welfare—tasks that require adequate funding for proper oversight and regulations.
Beyond immediate project delays, funding cuts can foster long-term skepticism about medical research among the public. Participants may become wary of enrolling in studies if they perceive a lack of sufficient oversight due to financial constraints. Furthermore, the cessation of collaborative efforts across multiple institutions can inhibit the pace of medical advancements. As research institutions grapple with budget cuts and funding shortages, the collective ability to safeguard patient interests and preserve ethical standards is increasingly jeopardized.
Impact on Patient Rights and Safety
The protection of patient rights and safety is at the forefront of medical research, necessitating rigorous oversight through proper funding and experienced personnel. Institutional Review Boards (IRBs) serve as the guardians of ethical principles in research, ensuring that studies adhere to established guidelines meant to protect participants. Cuts to funding diminish the capacity for thorough reviews of clinical trials, potentially allowing unethical practices or unsafe research protocols to prevail. The effectiveness of IRBs relies heavily on having the resources to conduct comprehensive evaluations, and without adequate funding, the safety net for patients begins to crumble.
Moreover, patient safety in research is not just a matter of compliance; it is a moral obligation that researchers and institutions must uphold. Historical events, such as the Tuskegee syphilis study, highlight the critical necessity of ethical oversight. Funding shortages can lead to a lack of personnel training and reduced ability to monitor ongoing trials effectively. Patients deserve assurance that they are participating in ethically approved research designed to prioritize their safety and welfare. With every dollar cut from research funding, the risk of priorities skewing towards expedience at the expense of ethics grows.
Role of Institutional Review Boards in Medical Research
Institutional Review Boards (IRBs) play a pivotal role in overseeing clinical research, ensuring that studies are conducted ethically while prioritizing participant welfare. IRBs meticulously review research protocols, considering potential risks and the processes for informed consent, among various other aspects. This review process is vital in maintaining the integrity of medical research and protecting the rights of participants. However, recent funding cuts have put immense pressure on these boards, limiting their ability to function effectively. It removes the financial resources necessary for thorough oversight and support in research projects.
Furthermore, IRBs are not just gatekeepers but also educators for researchers, guiding them to uphold clinical research ethics. The effective training and support they provide help to mitigate risks associated with clinical trials. Without adequate funding, the training of IRB members can suffer, leading to lapses in ethical standards during research reviews. As funding continues to dwindle, the fundamental role of IRBs in fostering a culture of ethical research is at risk, and with it, the safety and trust of research participants.
The Urgency of Sustaining Medical Research Funding
Staying committed to robust funding for medical research is crucial in easily mitigating risks to patient safety and upholding clinical ethics. Federal funding, particularly via organizations like the NIH, has historically played a substantial role in innovation and development within the healthcare sector. The cessation of grants and funding cuts translates directly to fewer resources available for crucial studies focused on diseases, treatments, and patient care practices. Consequently, the halt of funding disrupts the entire research ecosystem where patient safety and ethical scientific practices flourish.
In the face of such challenges, advocacy for continued investment in medical research is paramount. Supporting initiatives that reinforce funding integrity can ensure that studies remain ethically sound and effectively monitored. Delays in research due to funding cuts can have cascading effects on public health; thus, it is vital for stakeholders, including policymakers and the public, to recognize the importance of sustained funding for medical research as a means of protecting patient interests and fostering innovation.
Restoration of Trust through Ethical Research Practices
Trust in clinical research is deeply intertwined with effective IRB operations and the ethical conduct of research. When studies are conducted with the utmost integrity, patients feel assured that their rights are protected, and their contributions to science are valued. However, budget cuts jeopardize this trust. As researchers face limitations in resources, transparency and ethical practices may inadvertently suffer. A systemic failure to restore funding can lead to a cycle of mistrust among the very individuals that research aims to help.
To rebuild trust, it is essential that all parties involved in the medical research process—researchers, institutions, and funding agencies—commit themselves to transparency and clear communication. Patients must see tangible evidence that their safety and rights are priorities amidst funding crises. Open dialogue about the importance of ethical oversight, powered by sufficient funding, can help restore faith in clinical trials. Ensuring that IRBs can function diligently fosters a culture where patient welfare is front and center, enhancing trust in the larger research community.
Long-term Effects of Disrupted Research Initiatives
The long-term ramifications of disrupted funding for medical research extend far beyond immediate delays in studies. When critical studies are put on hold, the repercussions can span years or even decades, stalling the development of new treatments or medical interventions that could potentially save lives. The cumulative loss of innovations due to enduring funding cuts can result in a healthcare landscape that lags behind in addressing national health issues. Each halted study represents an opportunity lost to make advancements that are crucial for patient care.
Moreover, the reputational damage to research institutions can be profound. With diminished capacity to carry out significant studies, these institutions may find it challenging to attract future grants or collaborative opportunities. This creates a vicious cycle of reduced funding and decreased research output, leading to further stagnation. The eventual cost may not just be in scientific knowledge lost, but also in increased patient suffering as the potential benefits of research advancements remain unrealized.
Collaborative Approaches to Enhance Research Oversight
Collaboration among various research institutions as well as between IRBs can play a vital role in countering the adverse effects of funding cuts. Initiatives that promote sharing resources, knowledge, and best practices could help streamline the oversight process and ensure robust protections for research participants. By fostering community-wide partnerships, research teams can bolster their capacity to uphold ethical standards in the face of financial constraints.
Furthermore, collaborative approaches can enhance the overall efficiency of research oversight, reducing redundancies and facilitating a more coordinated response to ethics in research. Such synergy can help IRBs operate more effectively, even amid funding challenges. Building networks that connect institutions and their ethical oversight capacities can create a unified front to advocate for sustained funding and public trust in medical research.
Advancing Clinical Research Ethics Amidst Economic Challenges
Navigating clinical research ethics in the context of funding challenges requires innovative thinking and dedicated efforts from researchers and institutional leaders. Effective advocacy for ethical principles is vital, especially during financial constraints, ensuring that the rights and safety of participants remain paramount. Educational programs focused on upholding research ethics should be prioritized, ensuring that all stakeholders involved are trained to maintain high ethical standards despite funding limitations.
In addition, re-evaluating funding structures to prioritize ethical oversight can provide a more sustainable model for research funding. Strategies that capture the importance of ethics in funding proposals may support a financial framework that emphasizes not just scientific outcomes, but ethical conduct. By blending ethical imperatives with funding strategies, the research community can cultivate an environment where patient safety and ethical considerations remain unyielding priorities.
The Future of Medical Research in the Face of Fiscal Restraints
The future of medical research hangs in the balance as persistent fiscal restraints challenge the sustainability of essential studies. The ongoing cuts to federal funding threaten not just individual research projects but also long-term initiatives that could address pressing health challenges. As funding dwindles, collaboration is more necessary than ever to maximize the impact of every available dollar on patient-centered research. By pooling resources and fostering partnerships, the research community can work together to navigate the fiscal landscape.
Looking ahead, advocating for increased awareness and investment in medical research is crucial for the health of the nation. Future endeavors must prioritize ethical considerations while simultaneously bridging funding gaps. Only through sustained investment can we ensure that groundbreaking research continues to flourish, benefitting not just current patients, but also future generations who will rely on the advancements made today.
Frequently Asked Questions
What is the impact of funding cuts on medical research funding and patient safety?
Funding cuts to medical research can severely limit resources for essential oversight functions, including those provided by Institutional Review Boards (IRBs). When funding is reduced, IRBs struggle to maintain rigorous review processes that ensure patient safety in research. This can lead to inadequate monitoring of studies, increasing the risk of harm to participants and reducing public trust in medical research.
How do NIH funding cuts affect clinical research ethics?
NIH funding cuts can jeopardize the ethical oversight of clinical research by limiting the capacity of IRBs to uphold ethical standards. With fewer resources, IRBs may not be able to conduct thorough reviews or provide adequate training for researchers, which can compromise the integrity of informed consent processes and the overall ethical conduct of research involving human subjects.
What role do IRBs play in ensuring patient safety in medical research, especially during funding cuts?
IRBs are crucial for safeguarding patient safety by reviewing research proposals for ethical compliance and ensuring that participants are fully informed about the risks involved. During funding cuts, the ability of IRBs to perform these functions is weakened, which can lead to less oversight and increased risks for patients involved in clinical studies.
How do funding cuts to medical research affect the collaborative efforts in multi-site studies?
Funding cuts, such as those affecting SMART IRB, disrupt collaborative efforts by preventing the addition of new clinical sites and delaying research progress. This hampers the ability of various institutions to work together on multi-site studies, which can lead to postponed advancements in important medical research and negatively impact patient safety.
Why is adequate funding essential for the protection of patients in medical research?
Adequate funding is vital for the continued function of IRBs and other oversight bodies that ensure patient safety in medical research. Sufficient resources allow these organizations to perform necessary reviews, monitor studies effectively, and support researchers in maintaining ethical standards. Without this funding, the likelihood of ethical oversights and risks to participant safety increases significantly.
What can be the long-term consequences of NIH funding cuts on medical research and patient wellbeing?
Long-term NIH funding cuts can lead to a decline in the quality and quantity of medical research, resulting in slower development of new treatments and interventions. This can ultimately affect patient wellbeing since fewer studies may be conducted, hindering the discovery of effective therapies and the advancement of healthcare.
How do cuts in medical research funding undermine public trust in the research enterprise?
Cuts in medical research funding can exacerbate public skepticism and mistrust in the research community as halted studies may lead to negative outcomes for participants. This diminished trust can hinder future patient recruitment for trials, ultimately impacting the pace of medical advancements and the quality of patient care.
What measures are being taken to mitigate the impact of funding cuts on patient safety in medical research?
While funding cuts pose significant challenges, institutions are exploring alternative funding sources and prioritizing the ethical oversight of medical research. Collaborative efforts, such as sustaining SMART IRB with available resources, aim to maintain essential protections for study participants even amid financial constraints.
| Key Point | Description |
|---|---|
| Disruption of Research Oversight | The Trump administration’s freeze on $2 billion in federal grants has halted oversight mechanisms for medical studies. |
| Role of IRBs | Institutional Review Boards (IRBs) ensure the rights and safety of participants in clinical research. |
| Impact of Funding Cuts on IRBs | Research funding cuts threaten the integrity and function of IRBs, potentially leading to harm for study participants. |
| Public Trust | Halting ongoing studies undermines public trust in medical research and increases skepticism towards these initiatives. |
| Essential Work Continues | Despite funding cuts, ongoing support from institutions like Harvard Medical School aims to maintain critical research initiatives. |
Summary
The impact of funding cuts on medical research cannot be underestimated, as it fundamentally threatens the safety and oversight mechanisms designed to protect patients involved in studies. With the recent halt in federal research grants, essential programs like the SMART IRB face potential shutdowns, which could lead to delayed or stalled research projects. This disruption raises serious concerns about informed consent and participant safety, ultimately jeopardizing public trust in clinical research efforts. Moving forward, it is crucial that both policymakers and researchers work together to ensure the financial support needed to maintain the integrity of medical research and safeguard the health of individuals who volunteer for these studies.