Research into Alzheimer’s disease is paving the way for significant advancements in understanding this debilitating neurodegenerative disease. Led by pioneers like Beth Stevens, the study of microglial cells—the brain’s immune system—has unveiled their crucial role in maintaining cerebral health. These cells not only defend against pathogens but also participate in synaptic pruning, a process that, when disrupted, can lead to conditions like Alzheimer’s. Stevens’ groundbreaking findings have illuminated pathways for developing innovative Alzheimer’s treatments, enabling earlier diagnosis through new biomarkers. As the landscape of neurological research evolves, the hope for improving the lives of millions affected by Alzheimer’s continues to grow.
Investigations into age-related cognitive decline are increasingly vital as researchers seek to unravel the complexities surrounding dementia and related conditions. The focus on brain immunity and the behavior of glial cells, particularly in the context of synaptic functionality, underscores a transformative shift in our understanding of Alzheimer’s disease. The contributions of scientists like Beth Stevens highlight the importance of foundational research in revealing how disruptions beneath the surface of neural networks can lead to widespread neurological challenges. As the incidence of memory disorders rises, breakthroughs in the recognition and management of these ailments become ever more critical. Uniting the realms of basic science with practical applications may hold the key to novel strategies in combating age-associated cognitive impairments.
Understanding Microglial Cells and Their Role in Alzheimer’s Disease
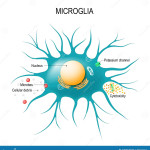
Microglial cells are the brain’s primary immune defense and play a crucial role in maintaining overall brain health. They are responsible for surveying brain activity, removing dead neurons, and regulating synaptic connections. Recent studies have illuminated how these cells contribute to the pathology of Alzheimer’s disease, particularly through the process of aberrant pruning. As these cells improperly prune synapses, they can disrupt communication between neurons, exacerbating the neurodegenerative effects of Alzheimer’s. Understanding this mechanism is pivotal, as amyloid plaques and tau tangles become key targets for potential therapeutic interventions.
Research by Beth Stevens at Boston Children’s Hospital has shown that microglia, when functioning optimally, participate in normal brain development and function. However, dysfunctional microglial activity is linked to various neurodegenerative diseases, including Alzheimer’s. By elucidating the relationship between microglial activity and Alzheimer’s, Stevens’ lab aims to pave the way for innovative treatments. This opens new avenues in Alzheimer’s research, suggesting that targeting microglial function may be as essential as targeting amyloid accumulation in the brain.
Beth Stevens’ Contributions to Alzheimer’s Research
Beth Stevens has emerged as a leading figure in Alzheimer’s research, particularly in the study of microglial cells. Her dedication to understanding how the brain’s immune system interacts with neurodegenerative diseases has transformed scientific perspectives. Stevens’ pioneering research has highlighted how microglial dysfunction contributes not only to Alzheimer’s but also to other cognitive disorders such as Huntington’s disease. Her work underscores the importance of exploring basic science to unlock potential breakthroughs in treatment methodologies for Alzheimer’s patients.
Stevens asserts that she was simply ‘following the science’ when her research began to uncover the complex role of microglial cells in brain health. Her findings have been instrumental, leading to the development of biomarkers that help in the early detection of Alzheimer’s disease. As the prevalence of Alzheimer’s continues to rise sharply in the aging population, the implications of her work extend beyond academic curiosity and hold promise for millions suffering from this devastating disease.
The Future of Alzheimer’s Treatments Based on Recent Discoveries
With the aging U.S. population, projections indicate that the incidence of Alzheimer’s may double by 2050, making research imperative. As Beth Stevens and her team continue to investigate the role of microglial cells, the potential for developing new Alzheimer’s treatments grows significantly. The understanding of how these immune cells can influence neurodegenerative processes opens the door for novel therapeutic strategies that could modify the disease course rather than just manage its symptoms.
Current Alzheimer’s treatments primarily focus on alleviating symptoms or slowing cognitive decline. However, Stevens’ research hints at a transformative approach that aims at repairing the underlying neural damage caused by improper microglial activity. By fostering an environment where microglia function correctly, scientists hope to create a new generation of Alzheimer’s treatments that not only target symptom relief but also address the root causes of the disease.
Innovative Biomarkers for Early Detection of Alzheimer’s Disease
One of the significant breakthroughs in Alzheimer’s research is the identification of biomarkers that can signal the disease in its early stages. Stevens’ work with microglial cells has enabled researchers to develop diagnostic tools that could detect changes in the brain’s immune response before the onset of clinical symptoms. These biomarkers are crucial as they facilitate earlier intervention, ultimately aiming to improve the quality of life for those predisposed to Alzheimer’s.
The promise of these biomarkers extends beyond mere detection; they may also help in monitoring the responsiveness to new treatments. As researchers unveil how microglial activity correlates with disease progression, these biomarkers could evolve to become essential components of Alzheimer’s disease management strategies, allowing healthcare providers to tailor interventions more effectively.
The Impact of Aging Population on Alzheimer’s Prevalence
As society experiences demographic shifts with an increasingly aging population, the prevalence of Alzheimer’s disease is becoming a pressing public health concern. The Alzheimer’s Association estimates that nearly 7 million Americans currently live with the disease, and that number is expected to rise dramatically in the coming decades. Understanding this trend is crucial for planning, funding, and implementing effective healthcare strategies to support individuals affected by Alzheimer’s.
This projected increase in Alzheimer’s cases will not only strain medical resources but also present significant emotional and social challenges for families and caregivers. By advancing our understanding of neurodegenerative diseases through pioneers like Beth Stevens, we can better prepare for this impending crisis. There is a clear need for increased funding for Alzheimer’s research to develop effective treatments and support systems that address the unique challenges associated with the disease’s progression.
Federal Support for Alzheimer’s Research Initiatives
Federal funding plays a pivotal role in advancing Alzheimer’s research, as highlighted by Beth Stevens’ own experience. The critical support from agencies like the National Institutes of Health has enabled researchers to pursue innovative studies and foster groundbreaking discoveries. This funding has been essential for facilitating research on microglial cells and their contributions to Alzheimer’s pathology.
Advocating for sustained and increased federal investment in Alzheimer’s research is essential for ensuring continued progress. As the societal burden of Alzheimer’s disease escalates, strategic funding can empower scientists like Stevens to uncover new avenues for treatment, diagnostics, and preventive strategies to combat this debilitating condition.
The Interplay Between Basic Science and Clinical Applications
Beth Stevens’ research underscores a fundamental truth: the interplay between basic science and clinical applications is vital for progress in medicine. By delving into the basic biological processes of microglial function, Stevens has illuminated pathways that may lead to innovative therapies for Alzheimer’s patients. Curiosity-driven research not only answers fundamental questions but also lays the groundwork for future clinical advancements.
This approach demonstrates how seemingly distant studies can converge on critical clinical applications. Stevens notes the importance of basic science in exploring neurobiological questions, which ultimately leads to a deeper understanding of Alzheimer’s disease mechanisms. The potential clinical impact of this research reiterates the necessity of continuous investment in basic scientific endeavors.
Neurodegenerative Diseases: Broader Implications Beyond Alzheimer’s
While Alzheimer’s disease often garners the most attention, the research being conducted by scientists like Beth Stevens also has broader implications for understanding other neurodegenerative diseases. Disorders such as Huntington’s disease and frontotemporal dementia also present significant challenges and share common pathways that could be influenced by microglial dysfunction.
By studying the role of microglial cells across various neurodegenerative conditions, researchers can uncover universal mechanisms that may inform treatment strategies. This comprehensive approach not only enriches our understanding of each individual disease but also fosters a collaborative atmosphere in the scientific community to tackle the complexities of brain health together.
Community Awareness and Support for Alzheimer’s Research
Increasing community awareness about Alzheimer’s and its research implications is crucial in combating the stigma associated with the disease. Educating the public about the importance of research conducted by pioneers like Beth Stevens can help garner support for ongoing studies and funding initiatives. Engaging communities in discussions around brain health and neurodegeneration fosters a collaborative environment for addressing these pressing health issues.
Support networks and community involvement can play a transformative role in the lives of those affected by Alzheimer’s. By building awareness, communities can mobilize volunteers, resources, and funding to support research efforts, feeding into the critical need for advancements in Alzheimer’s treatments and care initiatives. Awareness not only aids in research but also promotes a culture of understanding and compassion for those living with the disease.
Frequently Asked Questions
What role do microglial cells play in Alzheimer’s research?
Microglial cells are essential components of the brain’s immune system, playing a crucial role in Alzheimer’s research. They help clear away dead or damaged cells and prune synapses, which is vital for maintaining healthy brain function. However, in Alzheimer’s disease, aberrant pruning by microglia can contribute to neurodegeneration, highlighting their importance in understanding and developing potential Alzheimer’s treatments.
How do Alzheimer’s treatments target the brain’s immune system?
Alzheimer’s treatments are increasingly focusing on the brain’s immune system, particularly the role of microglial cells. Research led by scientists like Beth Stevens has shown that these cells can both protect and harm neurons. Current treatments aim to modulate microglial activity to enhance their protective functions while reducing harmful effects, paving the way for innovative approaches to combat neurodegenerative diseases.
Who is Beth Stevens and how is she related to Alzheimer’s research?
Beth Stevens is a prominent neuroscientist whose work has significantly advanced the field of Alzheimer’s research. She has focused on the role of microglial cells in brain health and disease, uncovering how their malfunction can contribute to neurodegenerative conditions like Alzheimer’s. Her research is pivotal in identifying new biomarkers and potential treatments for Alzheimer’s disease, transforming our understanding of the brain’s immune system.
What discoveries have been made regarding microglial cells in Alzheimer’s disease?
Recent discoveries in Alzheimer’s disease research highlight the dual role of microglial cells as both protectors and potential contributors to neurodegeneration. Aberrant pruning by these immune cells has been linked to Alzheimer’s pathology. Understanding these mechanisms is crucial for developing targeted therapies to modify microglial behavior, thus influencing the progression of neurodegenerative diseases.
How does impaired synaptic pruning by microglial cells affect Alzheimer’s progression?
Impaired synaptic pruning by microglial cells can lead to the accumulation of damaged neurons and dysfunctional synapses in the brain, accelerating the progression of Alzheimer’s disease. This aberrant activity contributes to neuroinflammation and cognitive decline, making it a key focus in Alzheimer’s research aimed at developing therapies that can restore healthy pruning functions.
What is the impact of aging on Alzheimer’s research and treatment strategies?
Aging significantly impacts Alzheimer’s research and treatment strategies, as the risk of developing neurodegenerative diseases increases with age. Current research emphasizes understanding the role of microglial cells in the aging brain and how their functions change over time. This understanding is critical for developing effective Alzheimer’s treatments that address both the underlying biology of aging and disease progression.
How can findings from Alzheimer’s research lead to earlier detection of the disease?
Findings from Alzheimer’s research, particularly those related to microglial cell function and synaptic pruning, can lead to the identification of new biomarkers. These biomarkers may allow for earlier detection of Alzheimer’s disease, enabling timely interventions that could potentially alter the disease’s trajectory and improve outcomes for those at risk.
| Key Point | Details |
|---|---|
| Research Focus | Neuroscientist Beth Stevens is studying microglial cells, the brain’s immune system, and how they influence diseases like Alzheimer’s. |
| Microglial Role | Microglia assist in clearing dead cells and pruning synapses, crucial for brain health and function. |
| Research Findings | Aberrant pruning by microglia can contribute to neurodegenerative diseases such as Alzheimer’s and Huntington’s disease. |
| Impact on Treatment | Stevens’ work lays the groundwork for developing new medicines and biomarkers that can help detect and treat Alzheimer’s earlier. |
| Future Projections | The number of Alzheimer’s cases in the U.S. is expected to double by 2050, increasing care costs significantly. |
Summary
Alzheimer’s Research is a rapidly evolving field, with scientists like Beth Stevens paving the way for innovative approaches to understanding and treating the disease. By investigating the roles of microglial cells, her work not only contributes to fundamental science but also holds promise for developing better diagnostics and therapies that could improve the lives of millions affected by Alzheimer’s. Ongoing research aims to address the challenges posed by this complex condition, highlighting the importance of sustained funding and exploration of new scientific avenues.